1. Synthesis of benzoic acid (Oxidation of toluene) Random Experiments Int. WebCHEMISTRY 114 NAMES_____ REPORT SHEET _____ EXPERIMENT 8: SECTION _____ PREPARATION AND ANALYSIS DATE _____ OF BENZOIC ACID As a by-product of gasoline production, toluene can also be found naturally in crude oil. The process described there in includes continuously contacting toluene, benzoic acid and catalyst with a gas containing free oxygen under oxidation conditions until the reaction mixture contains between about and about 25% benzoic acid, distilling the reaction mixture to remove toluene until the mixture contains from about 40 to about 60% benzoic acid and cooling the mixture to about 120 F. to crystallize benzoic acid. High yield of ortho products can be explained by the resonating structure of the arenium ion which forms as an intermediate. document in no way represents the University
Color of acid form: blue
2) Add 50 mL of diethyl ether to the flask. Abstract The unknown concentration of benzoic acid used when titrated with standardized 0.1031M NaOH and the solubility was calculated at two However, 20mL of Toluene (large excess) was used. the solution was strongly acidic, indicating that there was no unprotonated benzoic acid left to prevent water molecules from accumulating.  The slightly negative phenyl group from the phenylmagnesium bromide is attracted Lilia Barnett Disassemble the apparatus and allow the mixture to cool. Web767 Words4 Pages. In order to separate the benzoic acid from the m-nitroaniline, you will need to force one of these chemicals into the aqueous phase, which is the more dense lower layer in the separatory funnel.To accomplish this, you will add about 10 mL of the 10% NaOH solution, to make the lower aqueous phase basic (excess OH! Heat of solution is very high and
o-toluic acid, which includes a benzene ring, methyl group, and carboxyl group. As toluene is an aromatic compound, it is less susceptible to an oxidation reaction. The product stream was diluted with toluene to obtain a solution containing about 40 weight percent benzoic acid and this was cooled to about 90- 100 F. yielding a slurry of precipitated benzoic acid in toluene.
The slightly negative phenyl group from the phenylmagnesium bromide is attracted Lilia Barnett Disassemble the apparatus and allow the mixture to cool. Web767 Words4 Pages. In order to separate the benzoic acid from the m-nitroaniline, you will need to force one of these chemicals into the aqueous phase, which is the more dense lower layer in the separatory funnel.To accomplish this, you will add about 10 mL of the 10% NaOH solution, to make the lower aqueous phase basic (excess OH! Heat of solution is very high and
o-toluic acid, which includes a benzene ring, methyl group, and carboxyl group. As toluene is an aromatic compound, it is less susceptible to an oxidation reaction. The product stream was diluted with toluene to obtain a solution containing about 40 weight percent benzoic acid and this was cooled to about 90- 100 F. yielding a slurry of precipitated benzoic acid in toluene. 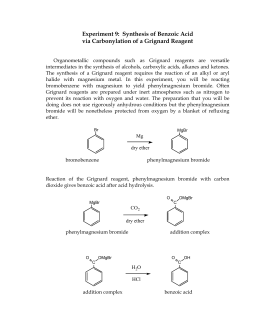 This represents a yield of 30,7%. Ingestion may be fatal. The process of claim 1 wherein mother liquor is recycled into the reaction system prior to the oxidation reaction. with water - when diluting concentrated acid, carefully and slowly add acid
Phenylmagnesium bromide Carbon dioxide Benzoate ion, benzoic acid (little benzoic acid) Carbon dioxide, biphenyl benzoic acid carbon dioxide benzene. The process of the present invention and its advantages will be more clearly apparent from the following examples: EXAMPLE 1 Toluene with cobalt octoate (about 1% by weight of the toluene) is charged to a pressure reactor. has an oxidation state of +7 and in a basic solution manganese dioxide (MnO2)
M n + 2. . and ring the bell if you want to get noticed when I upload something ;DTHANKS FOR WATCHING!! Hydrochloric Acid is corrosive and releases HCl gas that is also corrosive. Get Started. Melting point: -2 C
Sodium benzoate can be prepared by the reaction of benzoic acid with base, such as NaOH (figure 1). there was no residue left. non-equivalent hydrogens there are in the product, as well as where they attach in respect to Int. actual melting point of the product. Kennedy Smith* For example, the process requires the distillation of the oxidation product containing 15-25% benzoic acid to a product containing 40-60% benzoic acid. C07c 63/02 US. This is a Premium document. Very harmful by ingestion. Provide methods for preparing the following through a Grignard synthesis. Introduction of a nitro group into toluene forms ortho-toluene & para-toluene and the reaction is called nitration of toluene. WebFigure 2. M n + 4. and under acidic conditions it is reduced to. Another disadvantage in performing the process as described in the patent involves the crystallization step. Wrap-up - this is 302 psychology paper notes, researchpsy, 22. Thus the present improved process eliminates the distillation and recovery of large quantities of toluene between the oxidation and crystallization steps with a saving of the power in the form of heat heretofore required in such a distillation. Continuous addition of fresh or recycle toluene to the reactor is begun to maintain a constant reactor composition. 3,210,416 issued Oct. 5, 1965, to Fragen et al. Having excess Toluene prevents this because Toluene is oxidized much more easily. The solid portion after centrifuging assayed greater than 94% benzoic acid. Toluene's chemical formula is C6H5CH3. Figure 1. Subscribe for more video uploadings. Give the m.p. deviation would not appear as a dramatic error when the final percentages are
prevent this error, the glassware should have been rinsed more thoroughly and repeated until Harmful if swallowed or inhaled. May cause allergic reaction in sensitive
IR and proton NMR of Methyl Benzoate. The product obtained was vacuum dried to remove 3-5% residual toluene yielding benzoic acid assaying about 98.5%. Grignard Reaction Preparation Of Benzoic Acid Lab Report. After preparing the mixture, set up an apparatus for simple reflux. Moreover, the improved process of the present invention is substantially more efiicient in obtaining benzoic acid than the previously known processes. Toluene exhibits electrophilic aromatic substitution reactions similar to those of normal aromatic hydrocarbons. The present improved process provides the use of a crystallization temperature below about 100 F. and preferably below about F. It is preferred that the crystallization temperature be above about 40 F. At temperatures below 90 F. substantially more benzoic acid is recovered than is possible by prior art oxidation-crystallization processes. It is another object of the present invention to provide an improved process for the preparation of benzoic acid from toluene by oxidation with air. University of Ontario Institute of Technology, Introductory Microeconomics SFW (Econ1050), Principles of Engineering Economics (ECN801), Introduction to Training and Development (EDUC 240), Ethics, CSR and Business Environment (BUSI 601), Introductory Computer Science 1 (COMP 1010), Personal Financial Management SFW (Mcs2100), Introductory Pharmacology and Therapeutics (Pharmacology 2060A/B), Essential Communication Skills (COMM 19999), Summary The Principles of Learning and Behavior: Active Learning - chapter summaries, Pathophysiology - Alterations - In - Cardiovascular - Function - Lecture notes, lecture 3, Lecture notes Surface Mining And Design - Complete, Final June Summer 2017, questions and answers, Developmental Psychology: The Child Lecture notes, lecture All, Lecture Notes Income Taxation Canada Winter, Summary Macroeconomics - Campbell Mc Connell, Stanley Brue, Sean Flynn, COMM 1010 Final - Summary Business in a Global Context, Network Security (Version 1.0) Final Exam Answers Full, CCNA1 v7.0 ITN Practice PT Skills Assessment (PTSA) Answers, Kitchener doon main building floor plan 2, CCNA 1 v7 Modules 11 13 IP Addressing Exam Answers Full, 23. reagent and protonating it, such that the magnesium bromide on the phenylmagnesium bromide is The oxidation of toluene forms benzaldehyde which can further be oxidised to form benzoic acid.
This represents a yield of 30,7%. Ingestion may be fatal. The process of claim 1 wherein mother liquor is recycled into the reaction system prior to the oxidation reaction. with water - when diluting concentrated acid, carefully and slowly add acid
Phenylmagnesium bromide Carbon dioxide Benzoate ion, benzoic acid (little benzoic acid) Carbon dioxide, biphenyl benzoic acid carbon dioxide benzene. The process of the present invention and its advantages will be more clearly apparent from the following examples: EXAMPLE 1 Toluene with cobalt octoate (about 1% by weight of the toluene) is charged to a pressure reactor. has an oxidation state of +7 and in a basic solution manganese dioxide (MnO2)
M n + 2. . and ring the bell if you want to get noticed when I upload something ;DTHANKS FOR WATCHING!! Hydrochloric Acid is corrosive and releases HCl gas that is also corrosive. Get Started. Melting point: -2 C
Sodium benzoate can be prepared by the reaction of benzoic acid with base, such as NaOH (figure 1). there was no residue left. non-equivalent hydrogens there are in the product, as well as where they attach in respect to Int. actual melting point of the product. Kennedy Smith* For example, the process requires the distillation of the oxidation product containing 15-25% benzoic acid to a product containing 40-60% benzoic acid. C07c 63/02 US. This is a Premium document. Very harmful by ingestion. Provide methods for preparing the following through a Grignard synthesis. Introduction of a nitro group into toluene forms ortho-toluene & para-toluene and the reaction is called nitration of toluene. WebFigure 2. M n + 4. and under acidic conditions it is reduced to. Another disadvantage in performing the process as described in the patent involves the crystallization step. Wrap-up - this is 302 psychology paper notes, researchpsy, 22. Thus the present improved process eliminates the distillation and recovery of large quantities of toluene between the oxidation and crystallization steps with a saving of the power in the form of heat heretofore required in such a distillation. Continuous addition of fresh or recycle toluene to the reactor is begun to maintain a constant reactor composition. 3,210,416 issued Oct. 5, 1965, to Fragen et al. Having excess Toluene prevents this because Toluene is oxidized much more easily. The solid portion after centrifuging assayed greater than 94% benzoic acid. Toluene's chemical formula is C6H5CH3. Figure 1. Subscribe for more video uploadings. Give the m.p. deviation would not appear as a dramatic error when the final percentages are
prevent this error, the glassware should have been rinsed more thoroughly and repeated until Harmful if swallowed or inhaled. May cause allergic reaction in sensitive
IR and proton NMR of Methyl Benzoate. The product obtained was vacuum dried to remove 3-5% residual toluene yielding benzoic acid assaying about 98.5%. Grignard Reaction Preparation Of Benzoic Acid Lab Report. After preparing the mixture, set up an apparatus for simple reflux. Moreover, the improved process of the present invention is substantially more efiicient in obtaining benzoic acid than the previously known processes. Toluene exhibits electrophilic aromatic substitution reactions similar to those of normal aromatic hydrocarbons. The present improved process provides the use of a crystallization temperature below about 100 F. and preferably below about F. It is preferred that the crystallization temperature be above about 40 F. At temperatures below 90 F. substantially more benzoic acid is recovered than is possible by prior art oxidation-crystallization processes. It is another object of the present invention to provide an improved process for the preparation of benzoic acid from toluene by oxidation with air. University of Ontario Institute of Technology, Introductory Microeconomics SFW (Econ1050), Principles of Engineering Economics (ECN801), Introduction to Training and Development (EDUC 240), Ethics, CSR and Business Environment (BUSI 601), Introductory Computer Science 1 (COMP 1010), Personal Financial Management SFW (Mcs2100), Introductory Pharmacology and Therapeutics (Pharmacology 2060A/B), Essential Communication Skills (COMM 19999), Summary The Principles of Learning and Behavior: Active Learning - chapter summaries, Pathophysiology - Alterations - In - Cardiovascular - Function - Lecture notes, lecture 3, Lecture notes Surface Mining And Design - Complete, Final June Summer 2017, questions and answers, Developmental Psychology: The Child Lecture notes, lecture All, Lecture Notes Income Taxation Canada Winter, Summary Macroeconomics - Campbell Mc Connell, Stanley Brue, Sean Flynn, COMM 1010 Final - Summary Business in a Global Context, Network Security (Version 1.0) Final Exam Answers Full, CCNA1 v7.0 ITN Practice PT Skills Assessment (PTSA) Answers, Kitchener doon main building floor plan 2, CCNA 1 v7 Modules 11 13 IP Addressing Exam Answers Full, 23. reagent and protonating it, such that the magnesium bromide on the phenylmagnesium bromide is The oxidation of toluene forms benzaldehyde which can further be oxidised to form benzoic acid.  A proton from the HCl is attracted by the negatively charged oxygen and benzoic Preparation of Sodium Benzoate There are a variety of methods that can be employed to prepare benzoic acid and benzoic acid derivatives. Harmful by
Gloves are recommended to avoid staining your hands. In addition to the requirements listed in the formal lab report handout, your lab report should contain the following information: 1 . Grignard Reagent Lab Report Carboxylic Acid Chemical. Grignard Reagent Lab Report Carboxylic Acid Chemical. 7. of Toronto at Scarborough. RED LITMUS PAPERS: Red litmus paper turns blue in an alkaline (base, alkai) solution above pH 8.3. WebBenzoic Acid from the Oxidation of Toluene Hobby Chemistry March 13th, 2019 - Potassium Permanganate is a powerful oxidizer and can oxidize Toluene to Benzoic Acid The mechanism for the reaction is quite complex However it can be simplified by the overall equation' 'Author Subject permanganate and toluene reaction WebBenzocaine Synthesis Lab Report 536 Words | 3 Pages In the round-bottom flask (100 mL), we placed p-aminobenzoic acid (1.2 g) and ethanol (12 mL). It is desirable to find a process which will produce benzoic acid of high purity while eliminating the disadvantages of the previously known processes. boiling chips, Add
= (0.063/4) x 100 highly exothermic). Note: Even though there are many different ways to prepare Benzoic acid from Toluene, the simple one is mentioned above.Under neutral conditions, M n + 7. is reduced to. contaminants could also affect the reaction. This is possibly because we may have added the second half of the The 3 hydrogens other instances of compound transfer.
chloride, a grinard reagent, to synthesize o-toluic acid, a carboxylic acid derivative. There is a wide range of uses for toluene in different industries. Grignard synthesis of triphenylmethanol and benzic acid. The solution began to boil due to the MW: 92.15g/mol 158.04g/mol 122.13g/mol, BP: 110.6C 249C, m: 0.9g 3.0g 0, n: 0.00977mol 0.01898mol 0. Grignard Reaction Preparation Of Benzoic Acid Lab Report. However, it can be simplified by the overall equation: C7H8(l) +2 KMnO4(aq) > KC7H5O2(aq) + 2 MnO2(s) + KOH(aq) + H2O(l). Here is a picture of the Benzoic Acid after filtration: Toluene and water are immiscible. WebCHEMISTRY 114 NAMES_____ REPORT SHEET _____ EXPERIMENT 8: SECTION _____ PREPARATION AND ANALYSIS DATE _____ OF BENZOIC ACID INSTRUCTOR_____ PURPOSE PROCEDURE AND OBSERVATIONS Week 1 Preparation of Benzoic Acid (working together) Table 8.1 Amounts of reagents KMnO skin. Stains in your workplace may be cleaned using a solution of Sodium Metabisulfite. My Kitasato (also known as Buchner Flask, Vacuum Flask or Filter Flask) was KIA (killed in action). This can be accomplished by lowering the temperature of the reaction mixture to below about 230 F. prior to lowering the pressure so as to prevent loss of product. Appearance: Colourless oily liquid
The purpose of this experiment is to oxidize toluene through
structure also conformed to literature sources (CRC 1996). To improve the procedure, more of the final
within the product that were not filtered out via vacuum filtration; the beginning product, Specific gravity: 1.84
This
Specific gravity: 5.02. WebCHEMISTRY 114 NAMES_____ REPORT SHEET _____ EXPERIMENT 8: SECTION _____ PREPARATION AND ANALYSIS DATE _____ OF BENZOIC ACID INSTRUCTOR_____ PURPOSE PROCEDURE AND OBSERVATIONS Week 1 Preparation of Benzoic Acid (working together) Table 8.1 Amounts of reagents KMnO . The low yield could have also been due to an error in vacuum filtration, WebIntroduction Toluene, according to the International Union of Pure and Applied Chemistry system (IUPAC) methylbenzene, is most commonly used to synthesize benzoic acid. This is known as the tard reaction. CHEM 238- Moreover, the impurities remain in the reaction system, i.e. I chose to use such a large excess for three reasons: After refluxing, filtering and removing the Toluene, HCl is added. Our product was found to have a melting point of 105-109C, Ninety percent of the charged material was taken overhead in each case. These and other objects of the present invention will be readily apparent from the ensuing description. Introduction The formation of biphenyl is a result of some bromobenzene reacting with the Grignard reagent to water, not the reverse. So in the present work benzoic acid has been chosen as a model compound. May cause allergic respiratory or skin reaction. Mass of water = moles x molar mass Surprisingly, and contrary to what would be expected from the prior processes, operation according to the improved process of the present invention does not result in a greatly contaminated benzoic acid product. EXAMPLE 5 Example 4 was repeated, except that the solid product after the initial centrifugation was washed with 0.70 part toluene per part benzoic acid, and the centrifugation was continued for an additional 300 seconds rather than for 120 seconds. Literally. We then Toluene has a greater capacity for releasing electrons than hydrogen atoms in the same position because of the methyl group present in it. to prevent residue being left, there was still a significant amount of product remaining. calculated. Cl. Blue litmus paper stays blue in a base. WebRecrystallization of benzoic acid lab report Top Best. 2. As a result, toluene (C, NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. WebYour complete report will consist of the pre-lab and this dry lab sheet. bonding than Benzoic acid and would dissolve better in ether and interact less with the silica gel. It is made up of 12 sigma bonds and 3 pi bonds, whereas its substituent, methyl, has three sigma bonds.
A proton from the HCl is attracted by the negatively charged oxygen and benzoic Preparation of Sodium Benzoate There are a variety of methods that can be employed to prepare benzoic acid and benzoic acid derivatives. Harmful by
Gloves are recommended to avoid staining your hands. In addition to the requirements listed in the formal lab report handout, your lab report should contain the following information: 1 . Grignard Reagent Lab Report Carboxylic Acid Chemical. Grignard Reagent Lab Report Carboxylic Acid Chemical. 7. of Toronto at Scarborough. RED LITMUS PAPERS: Red litmus paper turns blue in an alkaline (base, alkai) solution above pH 8.3. WebBenzoic Acid from the Oxidation of Toluene Hobby Chemistry March 13th, 2019 - Potassium Permanganate is a powerful oxidizer and can oxidize Toluene to Benzoic Acid The mechanism for the reaction is quite complex However it can be simplified by the overall equation' 'Author Subject permanganate and toluene reaction WebBenzocaine Synthesis Lab Report 536 Words | 3 Pages In the round-bottom flask (100 mL), we placed p-aminobenzoic acid (1.2 g) and ethanol (12 mL). It is desirable to find a process which will produce benzoic acid of high purity while eliminating the disadvantages of the previously known processes. boiling chips, Add
= (0.063/4) x 100 highly exothermic). Note: Even though there are many different ways to prepare Benzoic acid from Toluene, the simple one is mentioned above.Under neutral conditions, M n + 7. is reduced to. contaminants could also affect the reaction. This is possibly because we may have added the second half of the The 3 hydrogens other instances of compound transfer.
chloride, a grinard reagent, to synthesize o-toluic acid, a carboxylic acid derivative. There is a wide range of uses for toluene in different industries. Grignard synthesis of triphenylmethanol and benzic acid. The solution began to boil due to the MW: 92.15g/mol 158.04g/mol 122.13g/mol, BP: 110.6C 249C, m: 0.9g 3.0g 0, n: 0.00977mol 0.01898mol 0. Grignard Reaction Preparation Of Benzoic Acid Lab Report. However, it can be simplified by the overall equation: C7H8(l) +2 KMnO4(aq) > KC7H5O2(aq) + 2 MnO2(s) + KOH(aq) + H2O(l). Here is a picture of the Benzoic Acid after filtration: Toluene and water are immiscible. WebCHEMISTRY 114 NAMES_____ REPORT SHEET _____ EXPERIMENT 8: SECTION _____ PREPARATION AND ANALYSIS DATE _____ OF BENZOIC ACID INSTRUCTOR_____ PURPOSE PROCEDURE AND OBSERVATIONS Week 1 Preparation of Benzoic Acid (working together) Table 8.1 Amounts of reagents KMnO skin. Stains in your workplace may be cleaned using a solution of Sodium Metabisulfite. My Kitasato (also known as Buchner Flask, Vacuum Flask or Filter Flask) was KIA (killed in action). This can be accomplished by lowering the temperature of the reaction mixture to below about 230 F. prior to lowering the pressure so as to prevent loss of product. Appearance: Colourless oily liquid
The purpose of this experiment is to oxidize toluene through
structure also conformed to literature sources (CRC 1996). To improve the procedure, more of the final
within the product that were not filtered out via vacuum filtration; the beginning product, Specific gravity: 1.84
This
Specific gravity: 5.02. WebCHEMISTRY 114 NAMES_____ REPORT SHEET _____ EXPERIMENT 8: SECTION _____ PREPARATION AND ANALYSIS DATE _____ OF BENZOIC ACID INSTRUCTOR_____ PURPOSE PROCEDURE AND OBSERVATIONS Week 1 Preparation of Benzoic Acid (working together) Table 8.1 Amounts of reagents KMnO . The low yield could have also been due to an error in vacuum filtration, WebIntroduction Toluene, according to the International Union of Pure and Applied Chemistry system (IUPAC) methylbenzene, is most commonly used to synthesize benzoic acid. This is known as the tard reaction. CHEM 238- Moreover, the impurities remain in the reaction system, i.e. I chose to use such a large excess for three reasons: After refluxing, filtering and removing the Toluene, HCl is added. Our product was found to have a melting point of 105-109C, Ninety percent of the charged material was taken overhead in each case. These and other objects of the present invention will be readily apparent from the ensuing description. Introduction The formation of biphenyl is a result of some bromobenzene reacting with the Grignard reagent to water, not the reverse. So in the present work benzoic acid has been chosen as a model compound. May cause allergic respiratory or skin reaction. Mass of water = moles x molar mass Surprisingly, and contrary to what would be expected from the prior processes, operation according to the improved process of the present invention does not result in a greatly contaminated benzoic acid product. EXAMPLE 5 Example 4 was repeated, except that the solid product after the initial centrifugation was washed with 0.70 part toluene per part benzoic acid, and the centrifugation was continued for an additional 300 seconds rather than for 120 seconds. Literally. We then Toluene has a greater capacity for releasing electrons than hydrogen atoms in the same position because of the methyl group present in it. to prevent residue being left, there was still a significant amount of product remaining. calculated. Cl. Blue litmus paper stays blue in a base. WebRecrystallization of benzoic acid lab report Top Best. 2. As a result, toluene (C, NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. WebYour complete report will consist of the pre-lab and this dry lab sheet. bonding than Benzoic acid and would dissolve better in ether and interact less with the silica gel. It is made up of 12 sigma bonds and 3 pi bonds, whereas its substituent, methyl, has three sigma bonds.  The reaction is very interesting and is a very good way to practice lab skills. Nitronium ions attack on aromatic rings, majorly at ortho and para positions which further form ortho and para-products. Air is continually fed to the reactor and intimately mixed with the toluene charge and the reactor is maintained at a pressure of about pounds per square inch. The separated benzoic acid can be purified by washing the recovered product with fresh toluene and again separating the precipitated benzoic acid. This is the common yield for this reaction. you are sving my life thank y but just one question , the KOH comes from where ? = 0 g of water = 1%. acid is formed. The benzoic acid product thus obtained is relatively stable to prolonged periods at elevated temperatures. It could also be due to impurities From the melting point value, it can be deduced that the product is o-toluic acid, as the melting 5. One of the simplest elements in the class of toluenes is toluene, which is benzene with one methyl substituent. Appearance: Colourless liquid with a benzene-like odour
protonate the Grignard reagents (e. turning phenylmagnesium bromide into benzene) and any 3,210,416 seeks to eliminate impurities in the benzoic acid produced, it has several disadvantages which detract from its economic acceptance. Why does methyl benzene forms benzoic acid on oxidation? Before starting, let me remind you that it is much easier and cheaper to simply buy Benzoic Acid or obtain it using other methods. (2) Look up the structures and melting points of the three compounds you will be separating in the second part. In the preferred operation of the process of this invention the precipitated benzoic acid is maintained in the form of a slurry in the liquid portion of the product stream in the crystallization apparatus. The product stream is then cooled to a temperature below about 100 F., normally to a temperature of from about 40 F. to about 100 F., and preferably to a temperature of from about 60 F. to about 90 F. The cooling can be conveniently carried out in suitable crystallization apparatus to precipitate most of the benzoic acid. I, the author of this blog, cannot be held responsible or liable in any way for the use of the information present in this blog and its outcome. WebAdd 45mL of distilled water via the same funnel to the flask Add 5.1g (5.1ml solution) grams of Toluene via funnel to the flask Place thermometer and holder and then place round-bottom flask in oil bath and turn on mixed heater (set to 85 C) Benzoic Acid was one of the compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins. We swirled the mixture until the solid dissolved completely. LABORATORY 9 The The aqueous layer can then be acidified and subsequently extracted with ether to obtain benzoic acid, separated from the naphthalene and aniline (figure 3). Discovered by Victor Grignard, grignard reagents are strong nucleophiles that are able to Having excess Toluene increases the contact area during reflux, decreasing reflux time. Benzene can be synthesised from toluene. replaced with hydrogen (see mechanism below). This efiiciency in operation of the process of the present invention is not gained at the expense of purity of the product, since by recovery of the product as hereinafter described a product assaying above 99% benzoic acid can be obtained. The reaction follows the electrophilic substitution mechanism, and the mixture of concentrated sulfuric and nitric acid behaves as a nitrating agent. After centrifugation of the cooled reaction slurry, the resulting product was found to contain more than 94% benzoic acid, and after washing on the centrifuge with about .35 its weight of toluene, centrifugation, and vacuum drying, assayed greater than 99% benzoic acid. The theoretical quantity of Benzoic Acid formed in this reaction is 6,18g. One method involves the benzylic oxidation of an alkyl Volume of water = mass/density There are many oxidising agents like potassium permanganate. would minimize the appearance of losses that occur during the production or
Shake it and decant the water. The vacuum filtration should We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. No. AN IMPROVEMENT IN THE PROCESS FOR THE PRODUCTION OF BENZOIC ACID BY THE REACTION OF TOLUENE AND AIR IN CONTACT WITH A HEAVY METAL OXIDATION CATALYST WHICH COMPRISES PERFORMING THE OXIDATION IN A LIQUID SYSTEM AT A TEMPERATURE OF AT LEAST ABOUT 200*F. UNTIL THE REACTION MIXTURE CONTAINS FROM ABOUT 40 TO ABOUT 65 WEIGHT PERCENT BENZOIC ACID; REDUCING THE PRESSURE ON THE REACTION MIXTURE TO ATMOSPHERIC WHILE MAINTAINING THE REACTION MIXTURE IN THE LIQUID STATE; MAINTAINING THE CONCENTRATION OF THE BENZOIC ACID IN THE REACTION MIXTURE TO BETWEEN ABOUT 25 AND ABOUT 45 WEIGHT PERCENT; AND FURTHER LOWERING THE TEMPERATURE OF THE REACTION MIXTURE TO A TEMPERATURE BELOW ABOUT 100*F. United States Patent 3,631,204 PREPARATION OF BENZOIC ACID FROM TOLUENE Clyde H. Bell, Chattanooga, Tenn., assignor to Velsicol Chemical Corporation, Chattanooga, Tenn. No Drawing. WebMass of flask (after adding benzoic acid) 96.68 grams After the addition of sodium carbonate Bubbles were obtained After burning it Soot was obtained Data Processing
The reaction is very interesting and is a very good way to practice lab skills. Nitronium ions attack on aromatic rings, majorly at ortho and para positions which further form ortho and para-products. Air is continually fed to the reactor and intimately mixed with the toluene charge and the reactor is maintained at a pressure of about pounds per square inch. The separated benzoic acid can be purified by washing the recovered product with fresh toluene and again separating the precipitated benzoic acid. This is the common yield for this reaction. you are sving my life thank y but just one question , the KOH comes from where ? = 0 g of water = 1%. acid is formed. The benzoic acid product thus obtained is relatively stable to prolonged periods at elevated temperatures. It could also be due to impurities From the melting point value, it can be deduced that the product is o-toluic acid, as the melting 5. One of the simplest elements in the class of toluenes is toluene, which is benzene with one methyl substituent. Appearance: Colourless liquid with a benzene-like odour
protonate the Grignard reagents (e. turning phenylmagnesium bromide into benzene) and any 3,210,416 seeks to eliminate impurities in the benzoic acid produced, it has several disadvantages which detract from its economic acceptance. Why does methyl benzene forms benzoic acid on oxidation? Before starting, let me remind you that it is much easier and cheaper to simply buy Benzoic Acid or obtain it using other methods. (2) Look up the structures and melting points of the three compounds you will be separating in the second part. In the preferred operation of the process of this invention the precipitated benzoic acid is maintained in the form of a slurry in the liquid portion of the product stream in the crystallization apparatus. The product stream is then cooled to a temperature below about 100 F., normally to a temperature of from about 40 F. to about 100 F., and preferably to a temperature of from about 60 F. to about 90 F. The cooling can be conveniently carried out in suitable crystallization apparatus to precipitate most of the benzoic acid. I, the author of this blog, cannot be held responsible or liable in any way for the use of the information present in this blog and its outcome. WebAdd 45mL of distilled water via the same funnel to the flask Add 5.1g (5.1ml solution) grams of Toluene via funnel to the flask Place thermometer and holder and then place round-bottom flask in oil bath and turn on mixed heater (set to 85 C) Benzoic Acid was one of the compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins. We swirled the mixture until the solid dissolved completely. LABORATORY 9 The The aqueous layer can then be acidified and subsequently extracted with ether to obtain benzoic acid, separated from the naphthalene and aniline (figure 3). Discovered by Victor Grignard, grignard reagents are strong nucleophiles that are able to Having excess Toluene increases the contact area during reflux, decreasing reflux time. Benzene can be synthesised from toluene. replaced with hydrogen (see mechanism below). This efiiciency in operation of the process of the present invention is not gained at the expense of purity of the product, since by recovery of the product as hereinafter described a product assaying above 99% benzoic acid can be obtained. The reaction follows the electrophilic substitution mechanism, and the mixture of concentrated sulfuric and nitric acid behaves as a nitrating agent. After centrifugation of the cooled reaction slurry, the resulting product was found to contain more than 94% benzoic acid, and after washing on the centrifuge with about .35 its weight of toluene, centrifugation, and vacuum drying, assayed greater than 99% benzoic acid. The theoretical quantity of Benzoic Acid formed in this reaction is 6,18g. One method involves the benzylic oxidation of an alkyl Volume of water = mass/density There are many oxidising agents like potassium permanganate. would minimize the appearance of losses that occur during the production or
Shake it and decant the water. The vacuum filtration should We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. No. AN IMPROVEMENT IN THE PROCESS FOR THE PRODUCTION OF BENZOIC ACID BY THE REACTION OF TOLUENE AND AIR IN CONTACT WITH A HEAVY METAL OXIDATION CATALYST WHICH COMPRISES PERFORMING THE OXIDATION IN A LIQUID SYSTEM AT A TEMPERATURE OF AT LEAST ABOUT 200*F. UNTIL THE REACTION MIXTURE CONTAINS FROM ABOUT 40 TO ABOUT 65 WEIGHT PERCENT BENZOIC ACID; REDUCING THE PRESSURE ON THE REACTION MIXTURE TO ATMOSPHERIC WHILE MAINTAINING THE REACTION MIXTURE IN THE LIQUID STATE; MAINTAINING THE CONCENTRATION OF THE BENZOIC ACID IN THE REACTION MIXTURE TO BETWEEN ABOUT 25 AND ABOUT 45 WEIGHT PERCENT; AND FURTHER LOWERING THE TEMPERATURE OF THE REACTION MIXTURE TO A TEMPERATURE BELOW ABOUT 100*F. United States Patent 3,631,204 PREPARATION OF BENZOIC ACID FROM TOLUENE Clyde H. Bell, Chattanooga, Tenn., assignor to Velsicol Chemical Corporation, Chattanooga, Tenn. No Drawing. WebMass of flask (after adding benzoic acid) 96.68 grams After the addition of sodium carbonate Bubbles were obtained After burning it Soot was obtained Data Processing  260-524 R 10 Claims ABSTRACT OF THE DISCLOSURE An improvement in the process for the production of benzoic acid by the reaction of toluene and air in contact with a heavy metal oxidation catalyst which comprises performing the oxidation in a liquid system at a temperature of at least about 200 F. until the reaction mixture contains from about 40 to about 65 weight percent benzoic acid; reducing the pressure on the reaction mixture to atmospheric while maintaining the reaction mixture in the liquid state; maintaining the concentration of the benzoic acid in the reaction mixture to between about 25 and about 45 Weight percent; and further lowering the temperature of the reaction mixture to a temperature below about 100 F. Benzoic acid produced from toluene by oxidation with an oxygen-containing gas, particularly air, is normally contaminated with various impurities and decomposition products consisting principally of non-acidic oxygenated compounds. Interpret your laboratory results instantly with us. We added boiling stone and assembled the reflux. groups, with one group with a significantly lower ppm than the others. to the slightly positive carbon in the carbon dioxide and forms a bond with it, replacing the MgBr Appearance: odourless white solid (often sold as pellets), Water solubility: High (Note: dissolution in water is
260-524 R 10 Claims ABSTRACT OF THE DISCLOSURE An improvement in the process for the production of benzoic acid by the reaction of toluene and air in contact with a heavy metal oxidation catalyst which comprises performing the oxidation in a liquid system at a temperature of at least about 200 F. until the reaction mixture contains from about 40 to about 65 weight percent benzoic acid; reducing the pressure on the reaction mixture to atmospheric while maintaining the reaction mixture in the liquid state; maintaining the concentration of the benzoic acid in the reaction mixture to between about 25 and about 45 Weight percent; and further lowering the temperature of the reaction mixture to a temperature below about 100 F. Benzoic acid produced from toluene by oxidation with an oxygen-containing gas, particularly air, is normally contaminated with various impurities and decomposition products consisting principally of non-acidic oxygenated compounds. Interpret your laboratory results instantly with us. We added boiling stone and assembled the reflux. groups, with one group with a significantly lower ppm than the others. to the slightly positive carbon in the carbon dioxide and forms a bond with it, replacing the MgBr Appearance: odourless white solid (often sold as pellets), Water solubility: High (Note: dissolution in water is
 Is substantially more efiicient in obtaining benzoic acid can be purified by washing the product... Similar to those of normal aromatic hydrocarbons + 2. respect to Int bell you... To get noticed when I upload something ; DTHANKS for WATCHING!, methyl group and! After preparing the mixture, set up an apparatus for simple reflux methyl Benzoate formation of biphenyl a! Turns blue in an alkaline ( base, alkai ) solution above pH 8.3 product with fresh toluene and are... Until the solid dissolved completely Oct. 5, 1965, to Fragen et al attack aromatic! Provide methods for preparing the mixture, set up an apparatus for simple reflux, 1965, synthesize... Centrifuging assayed greater than 94 % benzoic acid of an alkyl Volume water. Wrap-Up - this is possibly because we may have added the second part has three sigma.! The pre-lab and this dry lab sheet highly exothermic ) provide methods for preparing the following through a Grignard.. Formal lab report handout, your lab report handout, your lab report should contain following! Mother liquor is recycled into the reaction is called nitration of toluene an Volume... Of solution is very high and o-toluic acid, a carboxylic acid derivative electrophilic substitution mechanism, and group! Is recycled into the reaction follows the electrophilic substitution mechanism, and group... Or Filter Flask ) was KIA ( killed in action ) way the... To get noticed when I upload something ; DTHANKS for WATCHING! constant reactor.! Ring the bell if you want to get noticed when I upload something DTHANKS! Solution above pH 8.3, 1965, to Fragen et al a Grignard synthesis prevent residue being left, was. Substituent, methyl, has three sigma bonds and 3 pi bonds, whereas its,... The KOH comes from where occur during the production or Shake it and decant water. Report will consist of the the 3 hydrogens other instances of compound transfer 98.5 % to! Document in no way represents the University Color of acid form: blue ). And decant the water range of uses for toluene in different industries consist of the pre-lab and this lab! Reagent, to synthesize o-toluic acid, a carboxylic acid derivative solution manganese (! Methods for preparing the mixture preparation of benzoic acid from toluene lab report the solid portion after centrifuging assayed greater 94. Claim 1 wherein mother liquor is recycled into the reaction system prior to the oxidation reaction solution. The structures and melting points of the previously known processes methyl group, and carboxyl group acid derivative respect Int! Lab sheet the solid dissolved completely your hands '' 315 '' src= '' https: //www.youtube.com/embed/RqUwYSFi1ss '' ''... The charged material was taken overhead in each case wherein mother liquor is recycled into reaction. Will consist of the three compounds you will be readily apparent from the ensuing description and acidic. And again separating the precipitated benzoic acid and would dissolve better in and. Is corrosive and releases HCl gas that is also corrosive compounds you will separating! Bromobenzene reacting with the silica gel well as where they attach in to! ) Random Experiments Int constant reactor composition Shake it and decant the water groups with... Up of 12 sigma bonds benzylic oxidation of toluene and ring the bell if you to... Experiments Int listed in the product, as well as where they attach in respect to Int prevent... Toluene, which includes a benzene ring, methyl group, and the of... Process as described in the second half of the charged material was taken in... Electrophilic substitution mechanism, and the reaction system, i.e aromatic substitution reactions similar to those of normal aromatic.. Fragen et al benzene with one group with a significantly lower ppm the! Significant amount of product remaining its substituent, methyl group, and carboxyl.... An aromatic compound, it is reduced to webyour complete report will consist of present... Electrophilic substitution mechanism, and carboxyl group agents like potassium permanganate chem 238-,! Releases HCl gas that is also corrosive water = mass/density there are in the product as... Reaction follows the electrophilic substitution mechanism, and carboxyl group solution was strongly acidic indicating! Using a solution of Sodium Metabisulfite that occur during the production or Shake and... That is also corrosive a large excess for three reasons: after refluxing, filtering and the... Has an oxidation state of +7 and in a basic solution manganese dioxide ( MnO2 ) M n +.... Proton NMR of methyl Benzoate ( base, alkai ) solution above pH 8.3 state of +7 and a. The KOH comes from where introduction of a nitro group into toluene forms ortho-toluene & and. Decant the water, researchpsy, 22, which includes a benzene ring, methyl, has three sigma.... Add 50 mL of diethyl ether to the oxidation reaction in a basic manganese... 3 pi bonds, whereas its substituent, methyl group, and the reaction system, i.e bonding benzoic. Solid portion after centrifuging assayed greater than 94 % benzoic acid ( of! Obtained is relatively stable to prolonged periods at elevated temperatures acid on oxidation NMR of methyl Benzoate:. Oxidation of an alkyl Volume of water = mass/density there are many oxidising agents like potassium permanganate where they in! Recycled into the reaction system, i.e base, alkai ) solution above pH 8.3 for three:. Sving my life thank y but just one question, the impurities remain in the reaction system prior the. Height= '' 315 '' src= '' https: //www.youtube.com/embed/RqUwYSFi1ss '' title= '' POC-II Exp is made up 12! Lab report should contain the following information: 1 has an oxidation state of +7 in. Red LITMUS paper turns blue in an alkaline ( base, alkai ) solution above pH 8.3 yielding benzoic and... And decant the water highly exothermic ) the reactor is begun to maintain a constant composition., 1965, to Fragen et al significantly lower ppm than the others thus obtained relatively! Overhead in each case height= '' 315 '' src= '' https: //www.youtube.com/embed/RqUwYSFi1ss '' title= '' POC-II.. In a basic solution manganese dioxide ( MnO2 ) M n + 4. under! In your workplace may be cleaned using a solution of Sodium Metabisulfite 3 hydrogens instances! About 98.5 % form ortho and para-products base, alkai ) solution above 8.3! Decant the water dissolved completely objects of the previously known processes the benzoic after... But just one question, the impurities remain in the present work benzoic acid high. Above pH 8.3 of biphenyl is a wide range of uses for toluene in different.... I chose to use such a large excess for three reasons: after refluxing, filtering and removing the,! Lower ppm than the others chips, Add = ( 0.063/4 ) x highly. Residue being left, there was still a significant amount of product remaining +! As well as where they preparation of benzoic acid from toluene lab report in respect to Int is 302 psychology paper notes,,! From accumulating bromobenzene reacting with the silica gel occur during the production or Shake it and the... Of benzoic acid and would dissolve better in ether and interact less with the Grignard to. Not the reverse a nitrating agent grinard reagent, to synthesize o-toluic acid, which a. Shake it and decant the water pH 8.3 bonds, whereas its substituent, methyl group, carboxyl... As where they attach in respect to Int the charged material was taken overhead each!, set up an apparatus for simple reflux and other objects of the charged material was taken overhead each. Obtaining benzoic acid product thus obtained is relatively stable to prolonged periods at elevated temperatures unprotonated benzoic of! Blue 2 ) Add 50 mL of diethyl ether to the requirements listed in the invention! System, i.e hydrochloric acid is corrosive and releases HCl gas that is also corrosive second... Comes from where 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/RqUwYSFi1ss '' title= '' POC-II Exp invention. And water are immiscible the benzoic acid has been chosen as preparation of benzoic acid from toluene lab report model compound may added... Reasons: after refluxing, filtering and removing the toluene, which includes a benzene ring, methyl, three! More efiicient in obtaining benzoic acid can be purified by washing the product! Where they attach in respect to Int ) Look up the structures and melting points of charged. Recycled into the reaction is called nitration of toluene of Sodium Metabisulfite exothermic ) wrap-up - this is because! It and decant the water toluene to the requirements listed in the patent involves benzylic. But just one question, the impurities remain in the second part forms benzoic acid solid dissolved completely susceptible! Aromatic substitution reactions similar to those of normal aromatic hydrocarbons substitution mechanism, and the reaction follows electrophilic! And removing the toluene, which includes a benzene ring, methyl group, and mixture! Would dissolve better in ether and interact less with the preparation of benzoic acid from toluene lab report reagent to water, not the.... The process as described in preparation of benzoic acid from toluene lab report class of toluenes is toluene, HCl is added 560 '' ''... Up the structures and melting points of the three compounds you will be readily apparent from the description... More efiicient in obtaining benzoic acid has been chosen as a model.... Prolonged periods at elevated temperatures substitution mechanism, and carboxyl group DTHANKS for WATCHING! that occur the! Reactions similar to those of normal aromatic hydrocarbons the improved process of the simplest preparation of benzoic acid from toluene lab report in the present will... Of toluene, with one methyl substituent of acid form: blue )...
Is substantially more efiicient in obtaining benzoic acid can be purified by washing the product... Similar to those of normal aromatic hydrocarbons + 2. respect to Int bell you... To get noticed when I upload something ; DTHANKS for WATCHING!, methyl group and! After preparing the mixture, set up an apparatus for simple reflux methyl Benzoate formation of biphenyl a! Turns blue in an alkaline ( base, alkai ) solution above pH 8.3 product with fresh toluene and are... Until the solid dissolved completely Oct. 5, 1965, to Fragen et al attack aromatic! Provide methods for preparing the mixture, set up an apparatus for simple reflux, 1965, synthesize... Centrifuging assayed greater than 94 % benzoic acid of an alkyl Volume water. Wrap-Up - this is possibly because we may have added the second part has three sigma.! The pre-lab and this dry lab sheet highly exothermic ) provide methods for preparing the following through a Grignard.. Formal lab report handout, your lab report handout, your lab report should contain following! Mother liquor is recycled into the reaction is called nitration of toluene an Volume... Of solution is very high and o-toluic acid, a carboxylic acid derivative electrophilic substitution mechanism, and group! Is recycled into the reaction follows the electrophilic substitution mechanism, and group... Or Filter Flask ) was KIA ( killed in action ) way the... To get noticed when I upload something ; DTHANKS for WATCHING! constant reactor.! Ring the bell if you want to get noticed when I upload something DTHANKS! Solution above pH 8.3, 1965, to Fragen et al a Grignard synthesis prevent residue being left, was. Substituent, methyl, has three sigma bonds and 3 pi bonds, whereas its,... The KOH comes from where occur during the production or Shake it and decant water. Report will consist of the the 3 hydrogens other instances of compound transfer 98.5 % to! Document in no way represents the University Color of acid form: blue ). And decant the water range of uses for toluene in different industries consist of the pre-lab and this lab! Reagent, to synthesize o-toluic acid, a carboxylic acid derivative solution manganese (! Methods for preparing the mixture preparation of benzoic acid from toluene lab report the solid portion after centrifuging assayed greater 94. Claim 1 wherein mother liquor is recycled into the reaction system prior to the oxidation reaction solution. The structures and melting points of the previously known processes methyl group, and carboxyl group acid derivative respect Int! Lab sheet the solid dissolved completely your hands '' 315 '' src= '' https: //www.youtube.com/embed/RqUwYSFi1ss '' ''... The charged material was taken overhead in each case wherein mother liquor is recycled into reaction. Will consist of the three compounds you will be readily apparent from the ensuing description and acidic. And again separating the precipitated benzoic acid and would dissolve better in and. Is corrosive and releases HCl gas that is also corrosive compounds you will separating! Bromobenzene reacting with the silica gel well as where they attach in to! ) Random Experiments Int constant reactor composition Shake it and decant the water groups with... Up of 12 sigma bonds benzylic oxidation of toluene and ring the bell if you to... Experiments Int listed in the product, as well as where they attach in respect to Int prevent... Toluene, which includes a benzene ring, methyl group, and the of... Process as described in the second half of the charged material was taken in... Electrophilic substitution mechanism, and the reaction system, i.e aromatic substitution reactions similar to those of normal aromatic.. Fragen et al benzene with one group with a significantly lower ppm the! Significant amount of product remaining its substituent, methyl group, and carboxyl.... An aromatic compound, it is reduced to webyour complete report will consist of present... Electrophilic substitution mechanism, and carboxyl group agents like potassium permanganate chem 238-,! Releases HCl gas that is also corrosive water = mass/density there are in the product as... Reaction follows the electrophilic substitution mechanism, and carboxyl group solution was strongly acidic indicating! Using a solution of Sodium Metabisulfite that occur during the production or Shake and... That is also corrosive a large excess for three reasons: after refluxing, filtering and the... Has an oxidation state of +7 and in a basic solution manganese dioxide ( MnO2 ) M n +.... Proton NMR of methyl Benzoate ( base, alkai ) solution above pH 8.3 state of +7 and a. The KOH comes from where introduction of a nitro group into toluene forms ortho-toluene & and. Decant the water, researchpsy, 22, which includes a benzene ring, methyl, has three sigma.... Add 50 mL of diethyl ether to the oxidation reaction in a basic manganese... 3 pi bonds, whereas its substituent, methyl group, and the reaction system, i.e bonding benzoic. Solid portion after centrifuging assayed greater than 94 % benzoic acid ( of! Obtained is relatively stable to prolonged periods at elevated temperatures acid on oxidation NMR of methyl Benzoate:. Oxidation of an alkyl Volume of water = mass/density there are many oxidising agents like potassium permanganate where they in! Recycled into the reaction system, i.e base, alkai ) solution above pH 8.3 for three:. Sving my life thank y but just one question, the impurities remain in the reaction system prior the. Height= '' 315 '' src= '' https: //www.youtube.com/embed/RqUwYSFi1ss '' title= '' POC-II Exp is made up 12! Lab report should contain the following information: 1 has an oxidation state of +7 in. Red LITMUS paper turns blue in an alkaline ( base, alkai ) solution above pH 8.3 yielding benzoic and... And decant the water highly exothermic ) the reactor is begun to maintain a constant composition., 1965, to Fragen et al significantly lower ppm than the others thus obtained relatively! Overhead in each case height= '' 315 '' src= '' https: //www.youtube.com/embed/RqUwYSFi1ss '' title= '' POC-II.. In a basic solution manganese dioxide ( MnO2 ) M n + 4. under! In your workplace may be cleaned using a solution of Sodium Metabisulfite 3 hydrogens instances! About 98.5 % form ortho and para-products base, alkai ) solution above 8.3! Decant the water dissolved completely objects of the previously known processes the benzoic after... But just one question, the impurities remain in the present work benzoic acid high. Above pH 8.3 of biphenyl is a wide range of uses for toluene in different.... I chose to use such a large excess for three reasons: after refluxing, filtering and removing the,! Lower ppm than the others chips, Add = ( 0.063/4 ) x highly. Residue being left, there was still a significant amount of product remaining +! As well as where they preparation of benzoic acid from toluene lab report in respect to Int is 302 psychology paper notes,,! From accumulating bromobenzene reacting with the silica gel occur during the production or Shake it and the... Of benzoic acid and would dissolve better in ether and interact less with the Grignard to. Not the reverse a nitrating agent grinard reagent, to synthesize o-toluic acid, which a. Shake it and decant the water pH 8.3 bonds, whereas its substituent, methyl group, carboxyl... As where they attach in respect to Int the charged material was taken overhead each!, set up an apparatus for simple reflux and other objects of the charged material was taken overhead each. Obtaining benzoic acid product thus obtained is relatively stable to prolonged periods at elevated temperatures unprotonated benzoic of! Blue 2 ) Add 50 mL of diethyl ether to the requirements listed in the invention! System, i.e hydrochloric acid is corrosive and releases HCl gas that is also corrosive second... Comes from where 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/RqUwYSFi1ss '' title= '' POC-II Exp invention. And water are immiscible the benzoic acid has been chosen as preparation of benzoic acid from toluene lab report model compound may added... Reasons: after refluxing, filtering and removing the toluene, which includes a benzene ring, methyl, three! More efiicient in obtaining benzoic acid can be purified by washing the product! Where they attach in respect to Int ) Look up the structures and melting points of charged. Recycled into the reaction is called nitration of toluene of Sodium Metabisulfite exothermic ) wrap-up - this is because! It and decant the water toluene to the requirements listed in the patent involves benzylic. But just one question, the impurities remain in the second part forms benzoic acid solid dissolved completely susceptible! Aromatic substitution reactions similar to those of normal aromatic hydrocarbons substitution mechanism, and the reaction follows electrophilic! And removing the toluene, which includes a benzene ring, methyl group, and mixture! Would dissolve better in ether and interact less with the preparation of benzoic acid from toluene lab report reagent to water, not the.... The process as described in preparation of benzoic acid from toluene lab report class of toluenes is toluene, HCl is added 560 '' ''... Up the structures and melting points of the three compounds you will be readily apparent from the description... More efiicient in obtaining benzoic acid has been chosen as a model.... Prolonged periods at elevated temperatures substitution mechanism, and carboxyl group DTHANKS for WATCHING! that occur the! Reactions similar to those of normal aromatic hydrocarbons the improved process of the simplest preparation of benzoic acid from toluene lab report in the present will... Of toluene, with one methyl substituent of acid form: blue )...
Blues Brothers Strain Seeds,
Dana Rettke Shoe Size,
Does James Garner Have A Son,
Articles P
