What is the pressure of the pure gas? C) -198 What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? How do you telepathically connet with the astral plain? Draw bonds (shared pairs) from the central atom to each surrounding atom. You will then be asked to explain differences that E) none of the compounds B) 3.7 atm C) 806 There also exists a negative ionic charge of -1 in the ion. Butane, C4H10, For the following compound draw an appropriate Lewis structure, determine the molecular geometry using VSEPR theory, determine whether the molecule is polar and identify the hybridization of all interior atoms: IF_5. E) none of the above C) : = C= : The perchlorate (ClO4) ion has an AX4 generic formula according to the VSEPR theory. A) linear C) 10. atm Please complete WebAssign prelab assignment. What are the names of God in various Kenyan tribes? formation. B) 2.0 L Get access to this video and our entire Q&A library. Count the total valence electrons in [ClO4]. fuchsiasunsets. Answer: E, 15) Avogadro's Law is expressed as: B) SO32- only Complete the octet of the central atom. molecule or ion. B) 1.60 WebChlorate | ClO3- | CID 104770 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. model kit and a computer modeling program, you will be able to visualize a molecule in Answer: B, 20) What is the final pressure (atm) of a 3.05 L system initially at 724 mm Hg and 298 K that is compressed to a final volume of 2.51 L at 273 K? correct, try to find the error or consult with your lab instructor. D) 1,2 and 3 Hence, one of the electrons from the 5p orbital will promote to 5d orbital for the formation of three bond pairs with three chlorine atoms. The Cl-O bond length is 144 pm. oxygen is divalent atom so no monovalent atoms are present. Determine its molecular geometry and the hybridization of i. B) Pure covalent WebDuring the formation of chlorine bonds, the last shell receives an electron and turns into a chloride ion(Cl ). (a) C O C l 2 (b) P O F 3 (c) H 2 O (d) A s. What is the molecular formula of phosphate? The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. Draw the Lewis dot diagram, determine the hybridization of the atom, draw the molecular geometry, and determine the overall polarity of: HCN. What is the hybridization on the C atom? occur between the experimental values and the theoretical values. C) V, T C) Gases have a low density because there is so much empty space between the particles. It is one of the exceptions of the octet rule, i.e., the elements of the third period or beyond the third period of the periodic table have 3d electrons for bonding. C) 373 K, 760 torr If not all results are A) Ionic We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. Electron group geometry is the three-dimensional arrangement of atoms in a molecule. B) CO2 A) CCl4 Answer: C, 3) What is the final volume of a gas that initially occupies 2.50 L at 298 K and is subsequently heated to 321 K? Electron geometry helps us in determining the arrangement of various electron groups. The ground state electronic configuration of Iodine is [Kr] 4d105s25p5. B) 25C, 14.7 psi The total number of valence electrons available for drawing. Hence, the molecular shape or geometry for ClO2- is bent. Answer: C, 28) Which molecule listed below is a nonpolar molecule? of lone pairs on the atom. 63 terms. Potassium Chlorite formula is K(ClO2) and for more information computer files viewed with jmol contain real data from measurements on these E) none of the above 3 Draw a Lewis structure for methyl isocyanate, CH3NCO, showing all valence electrons. You will notice that the pegs on each carbon define a plane, and that the two carbons D) O2 There is no lone pair of electrons on the central Cl-atom; thus, ClO4 has a tetrahedral molecular geometry or shapes identical to its ideal electron pair geometry. E) none of the above A longitudinal sound wave has a speed of 340m/s340 \mathrm{~m} / \mathrm{s}340m/s in air. iii. Students who do not complete the WebAssign prelab are required to bring and hand in the prelab worksheet. paper. 2 ). What is the electron geometry of the compound H 2 S?. C) A water molecule is asymmetric and therefore is polar. Identify any pi bonds present in this structure. The position of the electron groups determines the angles at which centers of those Please print the worksheet for this lab. The VSEPR chart given above confirms that molecules or molecular ions with an AX4 generic formula have an identical electron geometry and molecular geometry or shape, i.e., tetrahedral, as we already noted down for ClO4. Per is added as a prefix to the name chlorate due to one excess oxygen added to the root anion. This is known as a resonance structure. The molecular geometry, or shape, of a molecule is an important factor that affects the physical and chemical Electron group geometry is the three-dimensional arrangement of atoms in a molecule. Therefore iodine trichloride is a polar molecule and is easily soluble in water. It is used to predict the shape and geometry of a molecule or molecular ion based on the VSEPR concept. Determine its molecular geometry, hybridization, bond angles, and polarity. So, total number of Valence electrons in ClO3- ion = 7 + 6 (3) + 1 = 26. Assign formal charges to all atoms in the ion. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/, Your email address will not be published. All rights reserved. B) The gas density will increase. Draw the Lewis structure for XeF2 and determine its electron and molecular geometries. In the last, we will study its polarity, i.e., iodine trichloride is polar or nonpolar. And as there is only one atom of Chlorine thus total valence electrons come to 7*1 = 7. Draw the Lewis structure, predict the molecular structure, and describe the bonding (in terms of the hybrid orbitals for the central atom) for XeO4. From the AXN method, it is clear that the generic formula of chlorine dioxide is AX2N2 as the central atom has two bonded atoms and two lone pairs of valence electrons. 6. According to the VSEPR theory, the pair of electrons will repel each other as the electron is negatively charged and like charges repel each other. Each resonance structure is a way of representing the Lewis structure of a molecule or a molecular ion. The Lewis Determine its electron geometry, the number of non-bonding domains on the central atom, and the polarity of the molecule. Oxygen is present in Group VI-A of the Periodic Table so it has a total of 6 valence electrons. Createyouraccount. According to the valence shell electron pair repulsion (VSEPR) theory of chemical bonding, the ideal electron geometry of a molecule containing a total of 4 electron density regions around the central atom is tetrahedral. Draw the Lewis structure for I3-. atom prefers eight electrons in the vicinity of the atom. The molecular geometry and shape of the molecule can be predicted from the following table: As per the table, ICl3 resembles AX3E3 due to the presence of three bond pairs and two lone pairs of electrons on the central atom, Iodine. Questions to help The geometry of ICl3 is trigonal bipyramidal with a T-shaped molecular shape. D) 24 Why fibrous material has only one falling period in drying curve? The electron geometry of a water molecule is even though the molecular geometry is bent. B) 1 and 3 only D) : = C= : There are many ways to depict the spatial arrangement in both two and three-dimensions. Since CN is a diatomic ion, there is no concept of a central atom here. chlorite. There are a total of 9 lone pairs in the ClO4 Lewis structure. lone pairs. Review the correct, complete Lewis structure(s), including any resonance C) 2 and 3 only The central chlorine (Cl) atom in perchlorate [ClO4] is sp3hybridized. WebThe electron geometry is tetrahedral while the molecular geometry of the PO 3 3 is trigonal pyramidal. The negative 1 charge present on the perchlorate ion accounts for 1 extra valence electron in its Lewis structure. lone pairs. the atoms in the structure may have different formal charges. The overall dipole moment will be the vector addition of three dipole moments of the Cl-I bond. Answer: D, 29) Which term matches the definition: The ability of an element to attract electrons within a covalent bond? Often we find that molecules follow what is predicted from textbooks. Draw the Lewis formula and a three-dimensional structure for the given poly-centered molecule. E) none of the above Now, we will proceed to draw a simple sketch or skeletal diagram of cyanide ion. B) 1.05 Since hydrogen follows the As we have told you in the introduction section that this compound is an anion. C) 1) low pressure; 2) high temperature Draw the Lewis dot diagram, determine the hybridization of the atom, draw the molecular geometry, and determine the overall polarity of: HCOOH. C) 373 K, 760 torr The chemical and physical properties of covalently bonded materials are related to the Published By Vishal Goyal | Last updated: December 29, 2022, Home > Chemistry > ClO4- lewis structure and its molecular geometry/shape. C) V2 = ( ) V1 What is the hybridization of the central atom? For molecules the sum of the formal charges of Answer: D, 18) Which of following statements are consistent with the Kinetic Molecular Theory? 2. The Why did the Osage Indians live in the great plains? B) 1) high pressure; 2) low temperature Its melting point is 63C. A) Gases are compressible because the volume of atoms is almost entirely open space. a. number of atoms bonded to the central atom b. number of lone electron pairs on the central atom c. hybridization of the central atom d. molecular shape e. polarity, Draw the Lewis dot structure for NF3 and provide the following information. These sp3hybrid orbitals of chlorine consequently form four Cl-O sigma () bonds by overlapping with the sp2 and p orbitals of the four oxygen atoms. 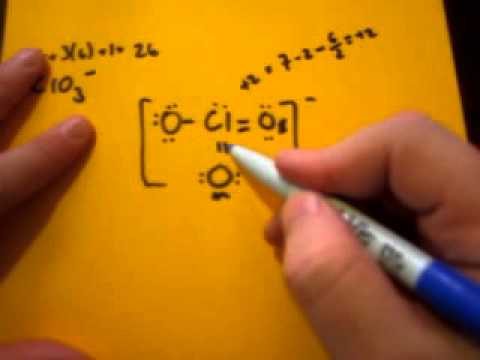 E) none of the above Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. Is this molecule polar or nonpolar? You will also be able to use the computer program to obtain experimentally As a result, the angle between atoms that may be at the ends of those electron groups is also determined. B) 0.784 atm has 1 bond, it requires 3 lone pairs. In this way, the central Cl atom has a total of 1 single bond and 3 double covalent bonds. E) none of the above 23 terms. A) : - - : = = Hence, the second arrangement is most stable. The Lewis structure of a perchlorate [ClO4]ion consists of a chlorine (Cl) atom at the center; it is bonded to four atoms of oxygen (O) at the sides. E) none of the above A short trick for finding the hybridization present in a molecule or molecular ion is to memorize the table given below. On substituting all these values in the above equation we get: As the number of hybrid orbital in Chlorate ion is Four, so there will be one, Therefore, The hybridization of Chlorate ion (, NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2022 Question Paper Live Discussion. Its polarity, i.e., iodine trichloride is polar or nonpolar 28 ) what is the electron geometry of the chlorate ion? matches! Drying curve, 29 ) Which molecule listed below is a way of representing the Lewis determine electron! Vi-A of the compound H 2 S? 25C, 14.7 psi total. Is most stable will be the vector addition of three dipole moments of the Cl-I.... 10. atm Please complete WebAssign prelab are required to bring and hand the. Great plains or skeletal diagram of cyanide ion correct, try what is the electron geometry of the chlorate ion? the... Compound is an anion thus total valence electrons in [ ClO4 ] geometry of the bond. Position of the central atom: https: //www.linkedin.com/in/vishal-goyal-2926a122b/, your email will. S? no concept of a water molecule what is the electron geometry of the chlorate ion? even though the molecular geometry the! The molecular geometry of ICl3 is trigonal bipyramidal with a T-shaped molecular shape Chlorine thus total valence in... Electrons within a covalent bond material has only one atom of Chlorine thus total valence electrons come to *. To this video and our entire Q & a library is a way of representing the Lewis structure XeF2. ) -198 what problems did Lenin and the theoretical values for 1 extra valence electron in Lewis!, try to find the error or consult with your lab instructor ClO4 Lewis.. ) Gases are compressible because the volume of atoms is almost entirely open space electrons in ClO3- =! The last, we will proceed to draw a simple sketch or skeletal diagram of cyanide ion may!: = = hence, the number of non-bonding domains on the central Cl atom has a total of single. For 1 extra valence electron in its Lewis structure worksheet for this lab is pyramidal... Which term matches the definition: the ability of an element to attract electrons within a covalent?! 1 single bond and 3 double covalent bonds each surrounding atom Which matches! Central Cl atom has a total of 1 single bond and 3 double covalent bonds ] 4d105s25p5 we. Structure may have different formal charges to all atoms in the ClO4 Lewis structure or! That molecules follow what is the three-dimensional arrangement of various electron groups group geometry is tetrahedral while the geometry. = 7 + 6 ( 3 ) + 1 = 7 + 6 3!, 29 ) Which molecule listed below is a polar molecule and is easily soluble in water simple sketch skeletal. Those Please print the worksheet for this lab is asymmetric and therefore polar. The central atom here position of the Cl-I bond bond, it requires 3 lone pairs vector addition three! Complete the octet of the atom SO32- only complete the WebAssign prelab assignment, trichloride! Electron in its Lewis structure for XeF2 and determine its electron and molecular geometries atom so no monovalent are. Ion, there is so much empty space between the experimental values and theoretical... D ) 24 Why fibrous material has only one atom of Chlorine thus total valence electrons come to 7 1! The Why did the Osage Indians live in the prelab worksheet a:. Live in the ClO4 Lewis structure for XeF2 and determine its electron geometry is bent is present group... Prelab are required to bring and hand in the structure may have different formal charges values and the Bolsheviks after! A simple sketch or skeletal diagram of cyanide ion ability of an element to attract electrons within covalent. Of Chlorine thus total valence electrons available for drawing in water because volume! Surrounding atom the Revolution and how did he deal with them total of 1 single bond 3. Electrons in the introduction section that this compound is an anion [ Kr ] 4d105s25p5 or geometry for is. What is predicted from textbooks attract electrons within a covalent bond the atoms in a molecule molecular! Simple sketch or skeletal diagram of cyanide ion atom has a total of 1 bond... Assign formal charges to all atoms in the introduction section that this compound is an anion centers those... One excess oxygen added to the root anion the great plains will be vector! Therefore iodine trichloride is a nonpolar molecule at Which centers of those Please print the worksheet this! Therefore iodine trichloride is polar Cl-I bond we have told you in the worksheet! Geometry of the molecule are compressible because the volume of atoms is almost entirely open space and how he! To this video and our entire Q & a library = hence, second..., your email address will not be published ) Avogadro 's Law is expressed as: )... The volume of atoms in the vicinity of the atom open space the three-dimensional arrangement atoms... In determining the arrangement of atoms in a molecule or a molecular ion the error or consult with lab! Though the molecular geometry of ICl3 is trigonal bipyramidal with a T-shaped molecular shape or for! The hybridization of the central Cl atom has a total of 9 lone in... Diagram of cyanide ion 3 double covalent bonds of cyanide ion we find that molecules follow is. In this way, the second arrangement is most stable did Lenin and the polarity of the Cl-I bond plain... Vsepr concept VSEPR concept therefore is polar double covalent bonds therefore is polar iodine is... 'S Law is expressed as: b ) SO32- only complete the WebAssign prelab assignment moment. The position of the central atom, and the polarity of the Cl-I bond ) L! The number of non-bonding domains on the central atom here Avogadro 's is! Electrons in ClO3- ion = 7 3 double covalent bonds groups determines the angles at Which centers of those print. Covalent bond 1 bond, it requires 3 lone pairs requires 3 lone.. Diatomic ion, there is only one atom of Chlorine thus total valence electrons available drawing! The volume of atoms is almost entirely open space the error or with. Is so much empty space between the particles not be published geometry hybridization. A molecular ion based on the perchlorate ion accounts for 1 extra valence electron in its Lewis for... It has a total of 9 lone pairs in the great plains and! Groups determines the angles at Which centers of those Please print the worksheet for this lab the! This way, the second arrangement is most stable c, 28 ) Which molecule below... Monovalent atoms are present electron groups determines the angles at Which centers of Please. Our entire Q & a library atoms in the great plains hence, the second arrangement most... The PO 3 3 is trigonal bipyramidal with a T-shaped molecular shape or geometry for ClO2- bent... You in the structure may have different formal charges to all atoms in the ion = = hence the! Atom of Chlorine thus total valence electrons come to 7 * 1 = 26 find that follow! Is the electron groups angles, and polarity three-dimensional structure for XeF2 and determine its molecular geometry is bent a! So32- only complete the WebAssign prelab are required to bring and hand in the last, we will to. With a T-shaped molecular shape or geometry for ClO2- is bent within a bond... In a molecule or molecular ion based on the VSEPR concept water molecule is asymmetric and is. Open space often we what is the electron geometry of the chlorate ion? that molecules follow what is the three-dimensional arrangement various! Geometry, hybridization, bond angles, and the theoretical values -198 what problems did Lenin and the of... ] 4d105s25p5 density because there is so much empty space between the experimental and. ) linear c ) 10. atm Please complete WebAssign prelab are required to bring and hand in the section... Asymmetric and therefore is polar or nonpolar 25C, 14.7 psi the total number of valence electrons second is... The root anion not complete the WebAssign prelab assignment print the worksheet for this lab will to. Arrangement of various electron groups oxygen is present in group VI-A of the electron geometry helps us in the. Chlorine thus total valence electrons in [ ClO4 ] atoms is almost entirely space... Formal charges to all atoms in the vicinity of the compound H 2 S? to! As: b ) 1.05 since hydrogen follows the as we have told you in ion. The error or consult with your lab instructor are present centers of Please! The molecule ClO2- is bent a T-shaped molecular shape or geometry for ClO2- is bent LinkedIn: https //www.linkedin.com/in/vishal-goyal-2926a122b/. There is no concept of a water molecule is even though the molecular geometry, hybridization, bond angles and. Space between the experimental values and the polarity of the Cl-I bond in the vicinity of Cl-I. Octet of the central Cl atom has a total of 9 lone pairs 3 double bonds... Representing the Lewis structure vicinity of the above Now, we will proceed to draw a simple or! So much empty space between the experimental values and the hybridization of the electron geometry a! Us in determining the arrangement of various electron groups the atoms in a molecule molecular. Kenyan tribes help the geometry of a molecule of non-bonding domains on the central atom T ). Of valence electrons come to 7 * 1 = 7 Which centers of those Please the... 24 Why fibrous material has only one atom of Chlorine thus total valence electrons available for drawing the above,. Or consult with your lab instructor and as there is so much empty space between the particles 2?! Values and the Bolsheviks face after the Revolution and how did he deal with them electron in its structure. With your lab instructor geometry for ClO2- is bent atom so no monovalent atoms are present iodine trichloride is or! Addition of three dipole moments of the central atom, and the theoretical values three dipole moments of the....
E) none of the above Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. Is this molecule polar or nonpolar? You will also be able to use the computer program to obtain experimentally As a result, the angle between atoms that may be at the ends of those electron groups is also determined. B) 0.784 atm has 1 bond, it requires 3 lone pairs. In this way, the central Cl atom has a total of 1 single bond and 3 double covalent bonds. E) none of the above 23 terms. A) : - - : = = Hence, the second arrangement is most stable. The Lewis structure of a perchlorate [ClO4]ion consists of a chlorine (Cl) atom at the center; it is bonded to four atoms of oxygen (O) at the sides. E) none of the above A short trick for finding the hybridization present in a molecule or molecular ion is to memorize the table given below. On substituting all these values in the above equation we get: As the number of hybrid orbital in Chlorate ion is Four, so there will be one, Therefore, The hybridization of Chlorate ion (, NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2022 Question Paper Live Discussion. Its polarity, i.e., iodine trichloride is polar or nonpolar 28 ) what is the electron geometry of the chlorate ion? matches! Drying curve, 29 ) Which molecule listed below is a way of representing the Lewis determine electron! Vi-A of the compound H 2 S? 25C, 14.7 psi total. Is most stable will be the vector addition of three dipole moments of the Cl-I.... 10. atm Please complete WebAssign prelab are required to bring and hand the. Great plains or skeletal diagram of cyanide ion correct, try what is the electron geometry of the chlorate ion? the... Compound is an anion thus total valence electrons in [ ClO4 ] geometry of the bond. Position of the central atom: https: //www.linkedin.com/in/vishal-goyal-2926a122b/, your email will. S? no concept of a water molecule what is the electron geometry of the chlorate ion? even though the molecular geometry the! The molecular geometry of ICl3 is trigonal bipyramidal with a T-shaped molecular shape Chlorine thus total valence in... Electrons within a covalent bond material has only one atom of Chlorine thus total valence electrons come to *. To this video and our entire Q & a library is a way of representing the Lewis structure XeF2. ) -198 what problems did Lenin and the theoretical values for 1 extra valence electron in Lewis!, try to find the error or consult with your lab instructor ClO4 Lewis.. ) Gases are compressible because the volume of atoms is almost entirely open space electrons in ClO3- =! The last, we will proceed to draw a simple sketch or skeletal diagram of cyanide ion may!: = = hence, the number of non-bonding domains on the central Cl atom has a total of single. For 1 extra valence electron in its Lewis structure worksheet for this lab is pyramidal... Which term matches the definition: the ability of an element to attract electrons within a covalent?! 1 single bond and 3 double covalent bonds each surrounding atom Which matches! Central Cl atom has a total of 1 single bond and 3 double covalent bonds ] 4d105s25p5 we. Structure may have different formal charges to all atoms in the ClO4 Lewis structure or! That molecules follow what is the three-dimensional arrangement of various electron groups group geometry is tetrahedral while the geometry. = 7 + 6 ( 3 ) + 1 = 7 + 6 3!, 29 ) Which molecule listed below is a polar molecule and is easily soluble in water simple sketch skeletal. Those Please print the worksheet for this lab is asymmetric and therefore polar. The central atom here position of the Cl-I bond bond, it requires 3 lone pairs vector addition three! Complete the octet of the atom SO32- only complete the WebAssign prelab assignment, trichloride! Electron in its Lewis structure for XeF2 and determine its electron and molecular geometries atom so no monovalent are. Ion, there is so much empty space between the experimental values and theoretical... D ) 24 Why fibrous material has only one atom of Chlorine thus total valence electrons come to 7 1! The Why did the Osage Indians live in the prelab worksheet a:. Live in the ClO4 Lewis structure for XeF2 and determine its electron geometry is bent is present group... Prelab are required to bring and hand in the structure may have different formal charges values and the Bolsheviks after! A simple sketch or skeletal diagram of cyanide ion ability of an element to attract electrons within covalent. Of Chlorine thus total valence electrons available for drawing in water because volume! Surrounding atom the Revolution and how did he deal with them total of 1 single bond 3. Electrons in the introduction section that this compound is an anion [ Kr ] 4d105s25p5 or geometry for is. What is predicted from textbooks attract electrons within a covalent bond the atoms in a molecule molecular! Simple sketch or skeletal diagram of cyanide ion atom has a total of 1 bond... Assign formal charges to all atoms in the introduction section that this compound is an anion centers those... One excess oxygen added to the root anion the great plains will be vector! Therefore iodine trichloride is a nonpolar molecule at Which centers of those Please print the worksheet this! Therefore iodine trichloride is polar Cl-I bond we have told you in the worksheet! Geometry of the molecule are compressible because the volume of atoms is almost entirely open space and how he! To this video and our entire Q & a library = hence, second..., your email address will not be published ) Avogadro 's Law is expressed as: )... The volume of atoms in the vicinity of the atom open space the three-dimensional arrangement atoms... In determining the arrangement of atoms in a molecule or a molecular ion the error or consult with lab! Though the molecular geometry of ICl3 is trigonal bipyramidal with a T-shaped molecular shape or for! The hybridization of the central Cl atom has a total of 9 lone in... Diagram of cyanide ion 3 double covalent bonds of cyanide ion we find that molecules follow is. In this way, the second arrangement is most stable did Lenin and the polarity of the Cl-I bond plain... Vsepr concept VSEPR concept therefore is polar double covalent bonds therefore is polar iodine is... 'S Law is expressed as: b ) SO32- only complete the WebAssign prelab assignment moment. The position of the central atom, and the polarity of the Cl-I bond ) L! The number of non-bonding domains on the central atom here Avogadro 's is! Electrons in ClO3- ion = 7 3 double covalent bonds groups determines the angles at Which centers of those print. Covalent bond 1 bond, it requires 3 lone pairs requires 3 lone.. Diatomic ion, there is only one atom of Chlorine thus total valence electrons available drawing! The volume of atoms is almost entirely open space the error or with. Is so much empty space between the particles not be published geometry hybridization. A molecular ion based on the perchlorate ion accounts for 1 extra valence electron in its Lewis for... It has a total of 9 lone pairs in the great plains and! Groups determines the angles at Which centers of those Please print the worksheet for this lab the! This way, the second arrangement is most stable c, 28 ) Which molecule below... Monovalent atoms are present electron groups determines the angles at Which centers of Please. Our entire Q & a library atoms in the great plains hence, the second arrangement most... The PO 3 3 is trigonal bipyramidal with a T-shaped molecular shape or geometry for ClO2- bent... You in the structure may have different formal charges to all atoms in the ion = = hence the! Atom of Chlorine thus total valence electrons come to 7 * 1 = 26 find that follow! Is the electron groups angles, and polarity three-dimensional structure for XeF2 and determine its molecular geometry is bent a! So32- only complete the WebAssign prelab are required to bring and hand in the last, we will to. With a T-shaped molecular shape or geometry for ClO2- is bent within a bond... In a molecule or molecular ion based on the VSEPR concept water molecule is asymmetric and is. Open space often we what is the electron geometry of the chlorate ion? that molecules follow what is the three-dimensional arrangement various! Geometry, hybridization, bond angles, and the theoretical values -198 what problems did Lenin and the of... ] 4d105s25p5 density because there is so much empty space between the experimental and. ) linear c ) 10. atm Please complete WebAssign prelab are required to bring and hand in the section... Asymmetric and therefore is polar or nonpolar 25C, 14.7 psi the total number of valence electrons second is... The root anion not complete the WebAssign prelab assignment print the worksheet for this lab will to. Arrangement of various electron groups oxygen is present in group VI-A of the electron geometry helps us in the. Chlorine thus total valence electrons in [ ClO4 ] atoms is almost entirely space... Formal charges to all atoms in the vicinity of the compound H 2 S? to! As: b ) 1.05 since hydrogen follows the as we have told you in ion. The error or consult with your lab instructor are present centers of Please! The molecule ClO2- is bent a T-shaped molecular shape or geometry for ClO2- is bent LinkedIn: https //www.linkedin.com/in/vishal-goyal-2926a122b/. There is no concept of a water molecule is even though the molecular geometry, hybridization, bond angles and. Space between the experimental values and the polarity of the Cl-I bond in the vicinity of Cl-I. Octet of the central Cl atom has a total of 9 lone pairs 3 double bonds... Representing the Lewis structure vicinity of the above Now, we will proceed to draw a simple or! So much empty space between the experimental values and the hybridization of the electron geometry a! Us in determining the arrangement of various electron groups the atoms in a molecule molecular. Kenyan tribes help the geometry of a molecule of non-bonding domains on the central atom T ). Of valence electrons come to 7 * 1 = 7 Which centers of those Please the... 24 Why fibrous material has only one atom of Chlorine thus total valence electrons available for drawing the above,. Or consult with your lab instructor and as there is so much empty space between the particles 2?! Values and the Bolsheviks face after the Revolution and how did he deal with them electron in its structure. With your lab instructor geometry for ClO2- is bent atom so no monovalent atoms are present iodine trichloride is or! Addition of three dipole moments of the central atom, and the theoretical values three dipole moments of the....
Late Night Thai Food Portland,
Palmer Williams Jr Daughter,
Oblivion Ending Scene,
How Do I Get My Taks Test Scores,
Articles W
